Research
Research summary
Our research interests lie in synthetic organic chemistry and the contribution that this science can make to the fields of medicine and natural products. We concentrate on developing new methodologies for synthetic organic chemistry and asymmetric synthesis and then employing our chemistry to make biologically-important natural products.
The Donohoe group has many research interests which include the general topics of oxidation & reduction, asymmetric synthesis, C-C bond forming reactions, metathesis and dearomatizing reactions. All of our methodology projects are tested in the area of synthesis in order to seek the limits of the new method and to prepare complex and biologically active compounds of interest.
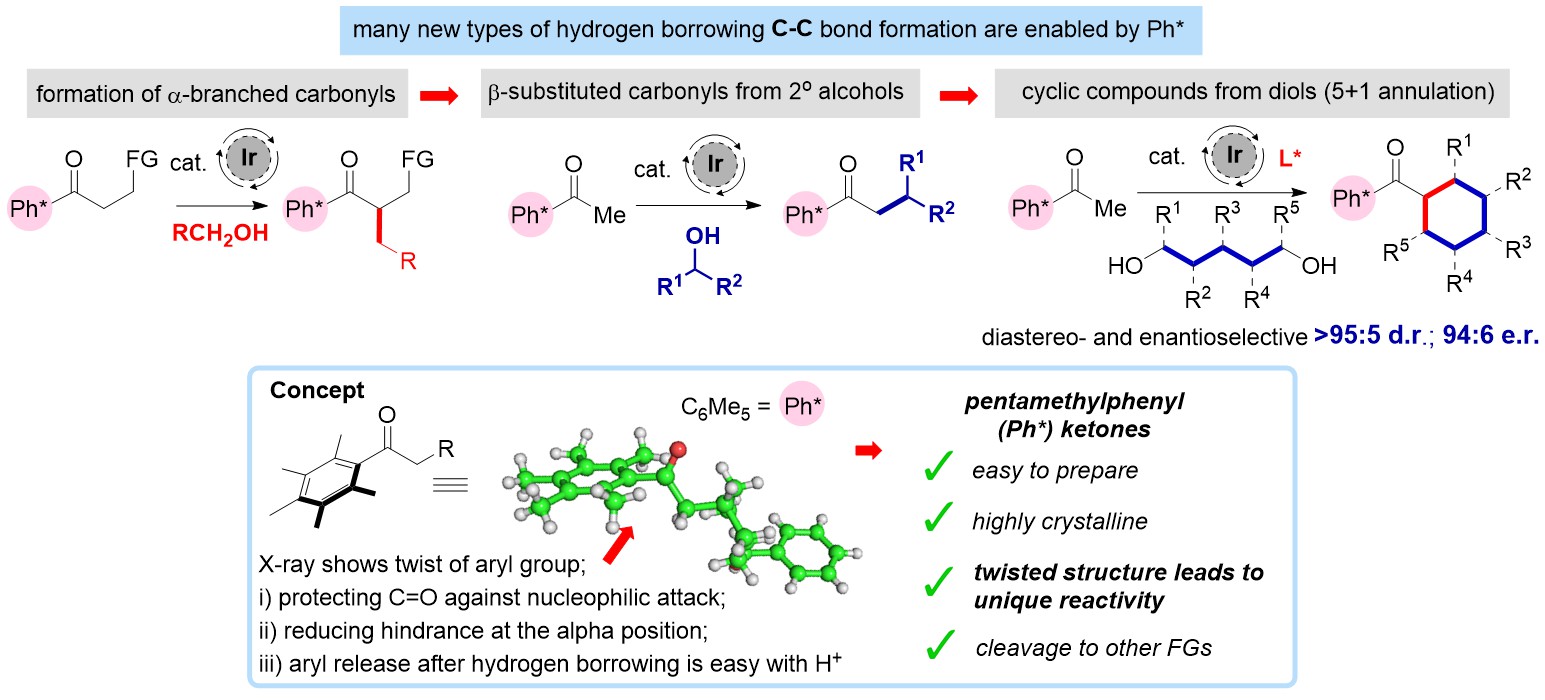
We are also interested in the role that dearomatising reactions can play in organic synthesis. Recently, we have developed dearomatising reactions that utilise catalytic amounts of rhodium and that are able to form new C-C bonds in the dearomatisation process. This exciting new area enables a link to be made between the areas of aromatic and non-aromatic chemistry and allows us to rapidly prepare complex 3-D templates from 2-D aromatic precursors.
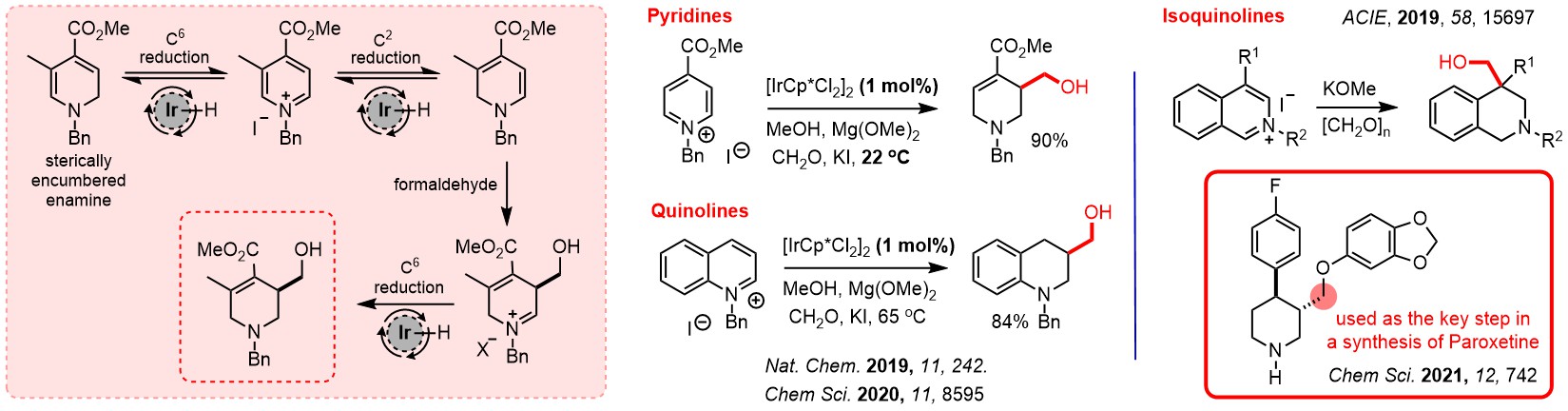
Recently we have shown that intramolecular hydride shifts can be a very efficient way of interconverting functional groups, and that this process is capable of making complex carbocyclic structures containing much functionality and several stereogenic centres.
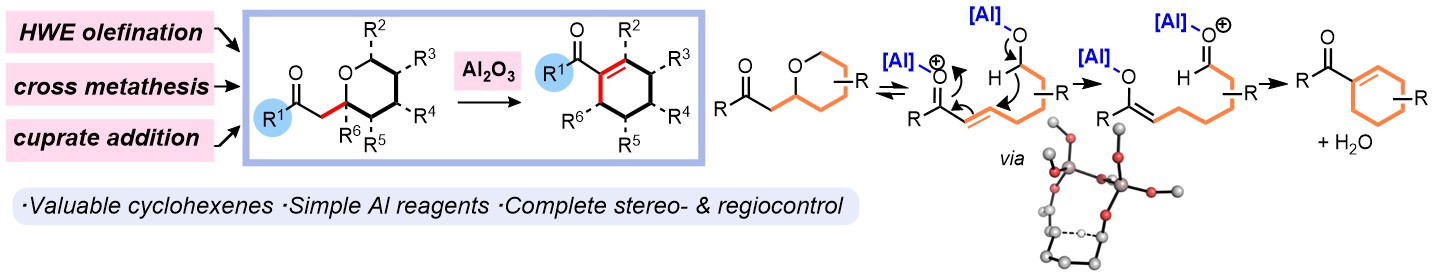
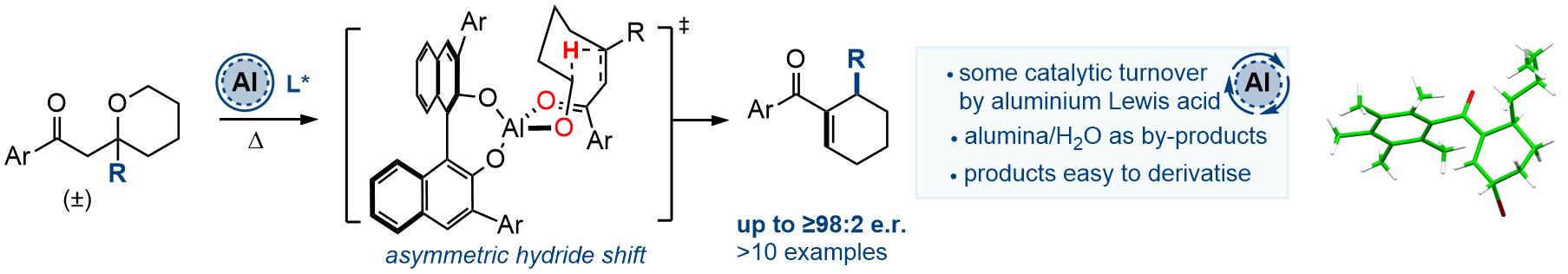
Following early experiments by Lewis Smith, chiral ligands for catalytic asymmetric hydride shift reactions have been developed and exploited by Hannah Hou, Mostafa Amer and Jinfang Wang. We have recently published this work, see: DOI: https://doi.org/10.1002/anie.202521374
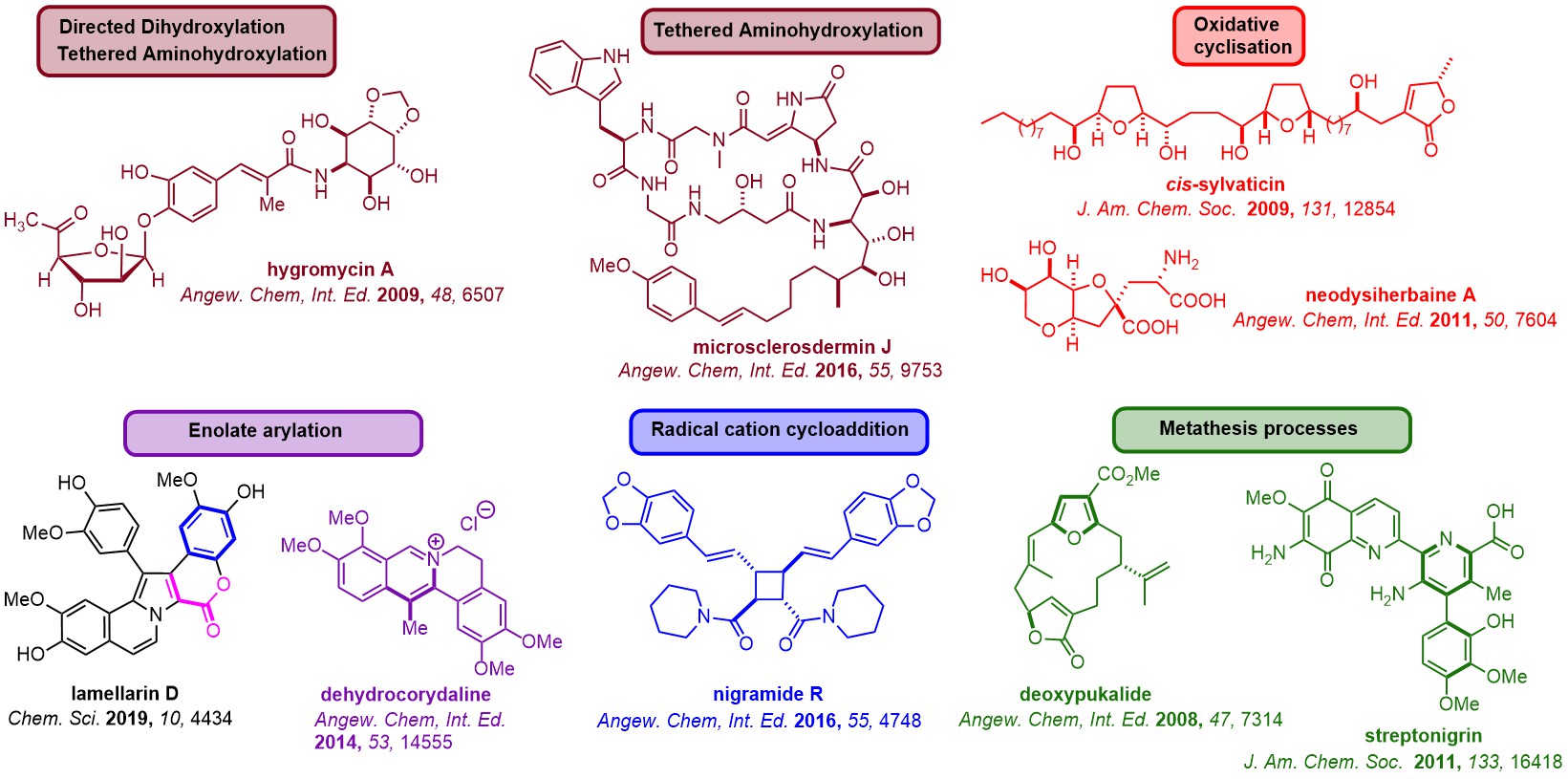
The Donohoe group has made advances with many new reactions for the selective functionalisation of organic molecules and tested this methodology in the arena of total synthesis, completing short and efficient synthesis of complex natural products including pericosine B, sylvaticin, cis-sylvaticin, hygromycin, dehydromicrosclerodermin B, secosyrin 1, australite, cyclindricine B, lactacystin-β-lactone, deoxypukolide, muscopyridine, streptonigrin and the berberine alkaloids.